
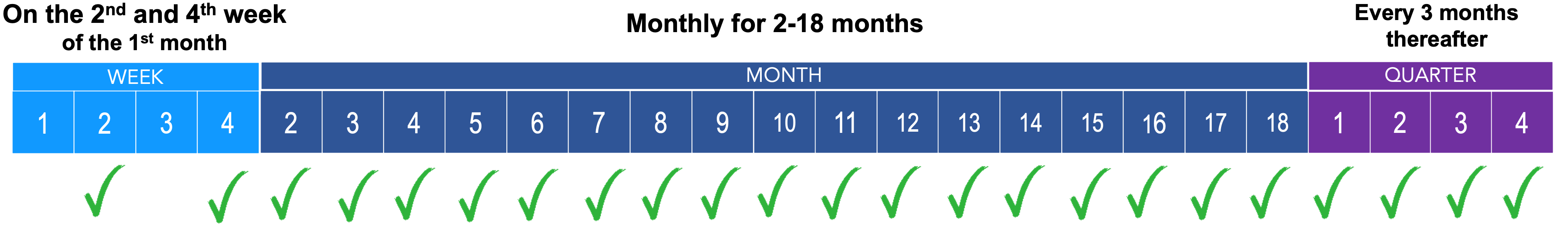
The JINARC® RMP aims to reduce the risk of severe and potentially fatal liver injury associated with the use of JINARC®.
This is done by:



Description of event

Patient information

Otsuka product involved e.g. Tolvaptan (JINARC®)

Primary reporter contact information
OPPI-Pharmacovigilance:
✓Email: oppi-pv@otsuka.com.ph
✓Mobile: +63999 8869 910
✓Phone: +632 8844 9266, +632 8888 6774
✓Fax: +632 8811 2279